Hello everyone. This blog is about us who don't know. Why we created this blog? we don't know...
This blog consist of 3 members who don't know. The leader of don't know is Abdul Rahman. He always say " I don't know" every time lecturer or us ask him anything. I don't know why he don't know. Next author is Shazwan Fahmi who is forced to join this group. The last author who was force to join this group is Krishoreraam.For ur information our leader is imported from Yemen and the youngest among us.
We are the students of MSU. Not Michigan State University, but Management and Science University, Shah Alam. We doing the bachelor in Pharmacy course. This is our second semester and also our first year. That's all about us. hahaha
Picture of Us: Shazwan, Krisho, Abdul Rahman( our leader from Yemen)
p/s: U all can still see the unwillingness from our face...hahaha
I Don't Know
Wednesday, 5 March 2014
What is a Protein? Learn about the 3D shape and function of macromolecules
Proteins are an important class of molecules found in all living cells. A protein is composed of one or more long chains of amino acids, the sequence of which corresponds to the DNA sequence of the gene that encodes it. Proteins play a variety of roles in the cell, including structural (cytoskeleton), mechanical (muscle), biochemical (enzymes), and cell signalling (hormones). Proteins are also an essential part of diet.
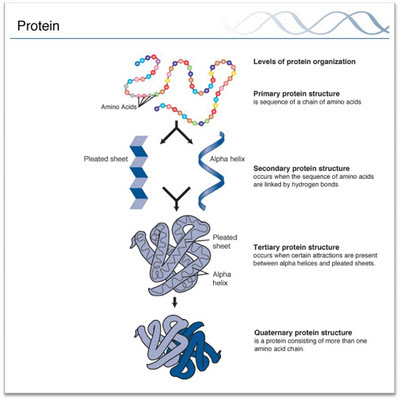
There are 4 levels of structure in
proteins
Primary, Secondary, Tertiary, Quaternary
Primary Structure
The linear sequence of
amino acids joined together by peptide bonds is termed the primary
structure of the protein
Val-Glu-Leu-Ala-Gly-Lys-Met
Peptide bond
- A protein is a linear sequence of amino acids linked together by peptide bonds
- Peptide bonds have a partial double-bond character (rigid & planar)
- Generally in the trans configuration
Secondary Structure
Refers to the regular folding of regions of the
polypeptide chain
Most common type of Secondary Structure:
(A) α-helix (a spiral)
(B) β-pleated sheet (folds)
Secondary Structural elements are stabilized by
extensive hydrogen bonding
(A) α-helix
- α-helix is a cylindrical, rod-like helical arrangement of the amino acids in the polypeptide chain which is maintained by hydrogen bonds parallel to the helix axis.In a α-helix there are 3.6 amino acids per turn of the helix covering a distance of 0.54 nm.
- Hydrogen bonds form between adjacent sections of polypeptides that are either running in the same direction (parallel β-pleated sheet) or in the opposite direction (anti parallel β-pleated sheet).
- Motifs (Super secondary structures) are produced by packing side chains from adjacent secondary elements close to each other.
Tertiary Structure
- Refers to the 3-dimesional (spatial) arrangement of all amino acids in the polypeptide chain.
- Biologically active (native) conformation stabilized by disulphide bonds, ionic bonds, hydrogen bonds and hydrophobic interactions.
- Domains are the functional and the three dimensional structural unit of a polypeptide. They are formed from combination of motifs
Quaternary Structure
· Proteins consists of more than one
polypeptide chain have quaternary structure. The poly peptides are held
together by covalent (disulphide bonds) or non covalent interactions
(hydrophobic interactions, electrostatic forces, hydrogen bonding)
Subscribe to:
Comments (Atom)